Microglial research is at the forefront of understanding how the brain’s immune system impacts neurodegenerative disorders like Alzheimer’s disease. These remarkable cells act as the brain’s guardians, constantly surveying and responding to injury or illness by facilitating synaptic pruning. However, recent studies have revealed that when microglial cells malfunction, their pruning processes can become destructive, contributing to the progression of conditions such as Alzheimer’s and Huntington’s disease. Pioneering work led by scientists like Beth Stevens has not only transformed our comprehension of microglial functions but has also laid the groundwork for innovative biomarkers and treatments targeting these critical pathways. As we delve deeper into microglial research, we edge closer to improving the lives of millions affected by Alzheimer’s, a daunting challenge faced by an estimated 7 million Americans.
Exploring the dynamics of glial cells, particularly microglia, unveils the intricate roles these brain-resident immune entities play in maintaining neurological health. Often referred to as the brain’s immune system, these cells are essential in safeguarding against pathological changes associated with neurodegenerative conditions. Their role in the delicate process of synaptic remodeling—where connections among neurons are formed and pruned—highlights their significance during normal brain development and in disease contexts. Advances in this field, spearheaded by thought leaders like Beth Stevens, contribute to a growing understanding of how dysfunctional microglial activity could exacerbate disorders such as Alzheimer’s disease. By shifting focus to microglial functions and their implications, researchers are paving the way for breakthroughs that could redefine therapeutic strategies for a range of debilitating diseases.
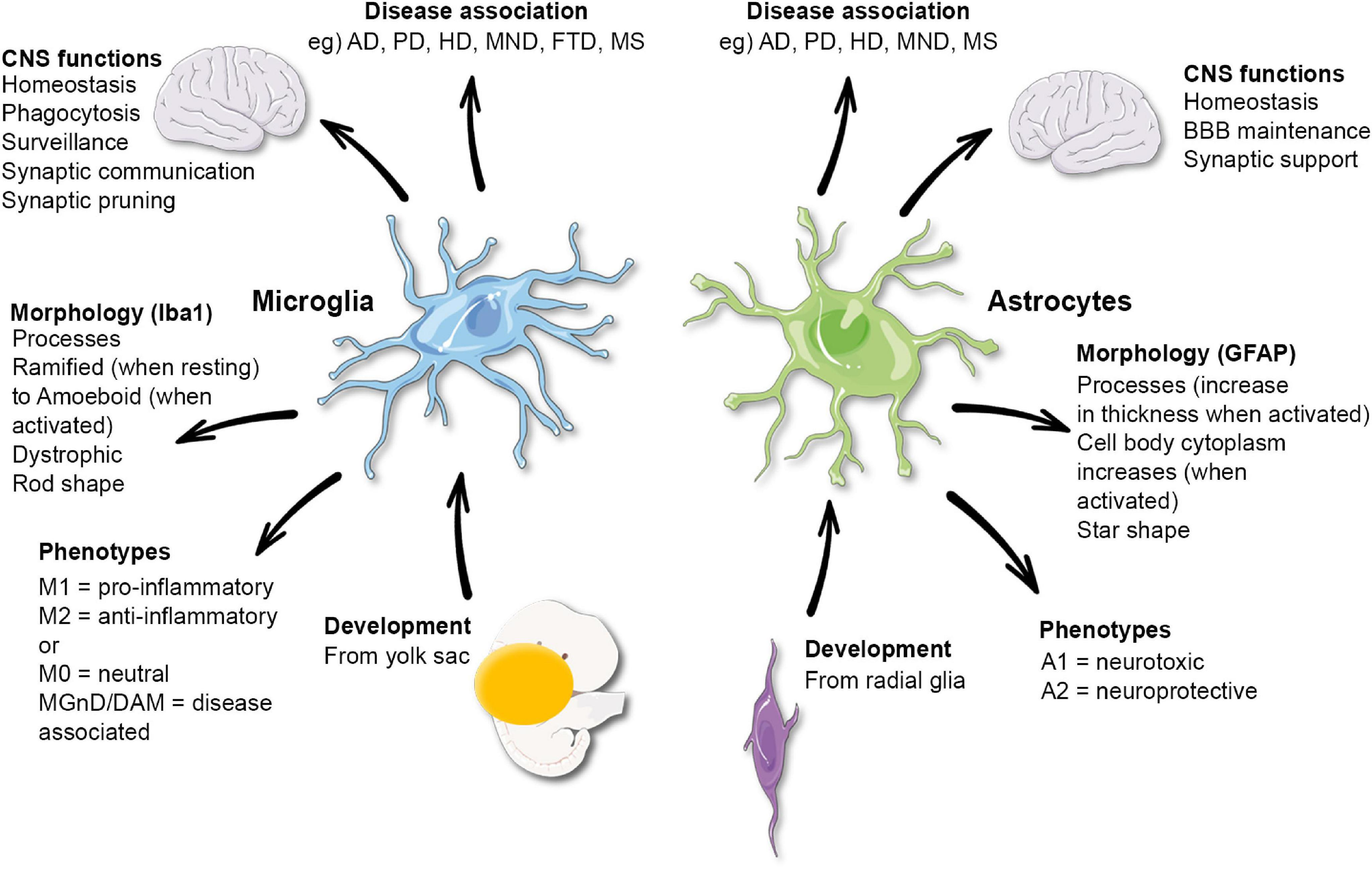
The Role of Microglial Research in Neurodegenerative Disorders
Microglial cells serve as the brain’s immune system, constantly surveying for injury or illness. Recent advancements in microglial research have shed light on their crucial role in neurodegenerative disorders such as Alzheimer’s disease. The Stevens Lab has been at the forefront of this research, revealing how microglia, when functioning correctly, clear out damaged cells and prune synapses to ensure proper neural communication. However, when this process goes awry, it can lead to aberrant synaptic pruning, contributing to the pathogenesis of diseases like Alzheimer’s and Huntington’s. Understanding microglial dynamics is not only pivotal in developing diagnostic tools but also in crafting new therapeutic strategies to combat these debilitating conditions.
This connection between microglial function and neurodegenerative diseases emphasizes the necessity of integrating basic science into clinical applications. As described by Beth Stevens, the groundwork laid by basic research is essential for understanding the complexities of these disorders. By investigating how microglial cells interact with neurotransmitters and modulate synaptic connections, researchers can uncover novel biomarkers that facilitate early diagnosis and treatment. Leveraging insights from this kind of research holds transformative potential for individuals affected by Alzheimer’s, thereby altering the course of care for millions.
Furthermore, the innovative discoveries stemming from microglial research highlight the importance of federal support. The financial backing from institutions like the National Institutes of Health has been instrumental in allowing scientists to dive deeper into how the brain’s immune system operates. Stevens’s work underscores the significance of federal grants in nurturing groundbreaking ideas, such as the concept of synaptic pruning driven by microglial activity. These insights not only pave the way for advanced therapeutic approaches but also challenge existing paradigms surrounding neurodegeneration, inspiring a new generation of researchers to explore the fundamental mechanisms at play.
Understanding the Brain’s Immune System
The brain’s immune system comprises various cells, with microglia being the primary players. These cells are not merely passive defenders; they play active roles in remodeling neural circuits during development and responding to injury. This dynamic function of microglia poses significant implications for understanding synaptic pruning, a process critical for maintaining cognitive functions. By investigating how microglia contribute to synaptic pruning, researchers glean insights into their involvement in disorders such as Alzheimer’s disease. For instance, the Stevens Lab’s research has significantly advanced the understanding of how irregular microglial activity can lead to the loss of synaptic integrity, which is a hallmark of neurodegeneration.
Research into the brain’s immune system has continuously unveiled the complexity of microglial involvement in both health and disease. For instance, improved techniques for visualizing and quantifying microglial function are revealing the intricate balance between synaptic support and elimination. This knowledge is crucial for designing interventions that can restore a proper immune response in the brain, potentially halting or even reversing neurodegenerative processes.
Interestingly, exploring the brain’s immune system also sheds light on the interplay between various neuroinflammatory processes. Cycles of inflammation can either maintain healthy neuronal function or contribute to synaptic dysfunction, underscoring the dual nature of microglial cells. For example, during neurodegeneration, persistent activation of microglia can lead to excessive inflammation, further damaging synapses. This phenomenon illustrates the critical need for targeted therapies that can modulate microglial activity without totally suppressing their protective roles. By understanding these cellular dynamics, scientists are laying the groundwork for innovative treatments that can better manage Alzheimer’s and related disorders.
Implications of Aberrant Synaptic Pruning
Aberrant synaptic pruning is emerging as a significant contributor to the pathophysiology of neurodegenerative diseases, particularly in Alzheimer’s disease. The ability of microglia to prune synapses is essential for normal brain development and function; however, disruptions in this process can lead to the elimination of necessary connections between neurons. Studies from the Stevens Lab have indicated that when microglia become overactive, they may indiscriminately remove synapses, leading to cognitive decline and memory loss. Understanding this maladaptive pruning situation has critical implications for developing novel interventions aimed at restoring synaptic health in affected individuals.
Moreover, the understanding of aberrant synaptic pruning has given rise to potential biomarkers for early detection of Alzheimer’s disease. A better grasp of how microglial activity correlates with synaptic loss can help researchers devise tests to assess the integrity of synaptic networks in patients, facilitating earlier and more precise interventions. This underscores the profound impact that microglial research has on the broader field of neurodegeneration, making it a focal point for future therapeutic strategies.
The implications of such findings extend beyond just Alzheimer’s disease; they resonate with various neurodegenerative disorders where synaptic connectivity is compromised. High-profile research, including that of Beth Stevens and associated collaborations, suggests that targeting microglial activity could offer new avenues for treatment. By potentially regulating the pruning process, therapies could preserve synaptic connections and combat cognitive decline across multiple neurological conditions. As researchers continue to unravel the complexities of synaptic pruning and microglial functionality, the prospect of developing groundbreaking treatments becomes increasingly promising.
Funding and Advancements in Neurobiological Research
Federal funding plays a vital role in advancing neurobiological research, particularly in understanding complex diseases like Alzheimer’s. Programs supported by the National Institutes of Health (NIH) have provided researchers, such as Beth Stevens, with the resources necessary to explore innovative ideas about the brain’s immune system. The initial support that established Stevens’s lab laid a crucial foundation that has now translated into transformative discoveries regarding microglial cells and their impact on neurodegenerative disorders. Such investments in research epitomize the importance of curiosity-driven science in uncovering the mysteries hidden within the brain and its numerous pathways.
Moreover, the continuous influx of funding has enabled researchers to utilize state-of-the-art technologies to study microglial behavior in real time. Advanced imaging techniques allow for the observation of microglial interactions with neurons, shedding light on their roles in synaptic pruning and responses to injury. This innovative approach has opened up new possibilities in understanding the etiology of neurodegenerative diseases like Alzheimer’s, emphasizing how crucial federal support is in fostering scientific breakthroughs that hold the potential to improve patient outcomes.
As the call for more substantial funding becomes increasingly evident, support for neurobiological research is essential to sustain momentum in this critical field. The advances made through collaborations between universities, hospitals, and federal agencies highlight the importance of a robust research ecosystem. Funding not only enables researchers to pursue answers to pressing questions but also facilitates collaboration that can lead to remarkable discoveries. The potential implications of this research go beyond academic understanding, aligning with the broader goal of improving healthcare for those impacted by Alzheimer’s and other debilitating neurodegenerative diseases.
The Journey of Curiosity-Driven Science
The journey of microglial research exemplifies the essence of curiosity-driven science, which often leads to unexpected yet groundbreaking discoveries. Beth Stevens’s exploration into the immune functions of microglia started from an initial interest in synaptic pruning during development. What began as an inquiry into how the brain’s immune system mediates neural connectivity ultimately unveiled critical avenues for understanding neurodegenerative disorders like Alzheimer’s disease. This trajectory affirms the premise that scientific exploration driven by genuine curiosity can unearth mechanisms that were previously unknown, providing insight into complex biological systems.
In this regard, curiosity-driven science not only fuels advances in academia but also has tangible implications for treatment development. By allowing scientists to explore hypotheses without the constraints of expected outcomes, significant revelations can emerge that challenge existing paradigms. For instance, early research into microglial functionality led to transformative insights about their role in synaptic health during Alzheimer’s disease, reinforcing the value of supporting inquisitive minds in the pursuit of understanding the brain.
Moreover, fostering an environment that encourages curiosity-led inquiries can inspire the next generation of researchers to think outside conventional frameworks. This encourages a culture of innovation that drives the enhancement of methodologies in neuroscience. It cultivates an ecosystem that not only values the end results but also appreciates the process of scientific discovery itself. As illustrated by Stevens’ journey, a commitment to exploring the unknown can lead to breakthroughs that significantly advance our ability to diagnose and treat neurodegenerative diseases, ultimately improving the quality of life for countless individuals.
New Horizons in Alzheimer’s Treatment and Detection
Recent advancements in microglial research open new horizons in the treatment and detection of Alzheimer’s disease. As new findings emerge from the Stevens Lab, pathways to developing targeted therapies aimed at correcting the malfunction of microglia show promise. With the understanding that aberrant synaptic pruning is a contributing factor to Alzheimer’s, therapies designed to modulate microglial activity could potentially restore lost synaptic connections, rejuvenating neural communication and memory functions. This shift from traditional approaches to more nuanced immune-targeting strategies could revolutionize standards of care for the millions affected by this progressive disorder.
In addition to therapeutic advancements, progress in understanding microglial involvement in disease mechanisms also aids in refining diagnostic tools. Researchers are keenly developing biomarkers linked to microglial activity that could allow for earlier detection of Alzheimer’s disease. Earlier diagnosis, in turn, enables timely intervention, which is crucial in managing symptoms and prolonging patient autonomy. Thus, the implications of ongoing microglial research in both treatment and diagnosis could substantially enhance outcomes in Alzheimer’s patients, marking a significant step forward in combating this debilitating disease.
The potential for microglial research to lead to new treatment modalities reflects a broader trend in neurology towards precision medicine. By identifying specific microglial profiles associated with various stages of Alzheimer’s, treatment can be more personalized, targeting the unique aspects of an individual’s disease progression. This personalized approach aligns with contemporary medical trends prioritizing tailored therapies over one-size-fits-all solutions. As researchers like Stevens continue to uncover the complexities of the brain’s immune responses, the potential for transformative impacts on the approach to Alzheimer’s disease becomes increasingly clear, offering hope for more effective management strategies.
Beth Stevens: A Pioneer in Neuroscience
Beth Stevens stands out as a pioneering figure in the realm of neuroscience, particularly with her innovative work on microglial research. Her groundbreaking exploration has shifted paradigms regarding how we understand the brain’s immune system, specifically in its role in synaptic pruning and the progression of neurodegenerative diseases like Alzheimer’s. Recognized for her contributions, Stevens was awarded the MacArthur “genius” grant, which highlights not just her scientific acumen but the transformative nature of her research. As an associate professor at Harvard Medical School, she continues to bridge fundamental research with practical applications that can directly impact patient care.
Stevens’s insightful findings have drawn attention to the inner workings of the brain, emphasizing the complexity of microglial interactions within neural networks. Her determination to follow the science and foster curiosity-driven research exemplifies the kind of innovative thinking that propels neuroscience forward. By advocating for the importance of microglial cells in maintaining brain health and their implications in neurodegenerative disorders, she has opened new pathways for research and therapeutic strategies, reinforcing her status as a leader in the field.
Moreover, her collaborative approach underscores the necessity of multidisciplinary efforts in scientific research. By working alongside teams at Boston Children’s Hospital and the Broad Institute of MIT and Harvard, Stevens has been able to leverage diverse expertise to accelerate discoveries. This model of collaboration not only enhances scientific inquiry but fosters an inclusive environment that encourages innovative solutions to complex problems in neurobiology. As the community continues to recognize the importance of microglial research, Stevens’s influence persists in shaping new directions in the understanding and treatment of conditions like Alzheimer’s disease.
Frequently Asked Questions
What is the role of microglia in Alzheimer’s disease?
Microglia are critical components of the brain’s immune system, actively involved in patrolling for illness and injury. In the context of Alzheimer’s disease, they help clear out dead neurons and contribute to synaptic pruning. However, aberrant microglial activity can lead to excessive synaptic pruning, which may promote the progression of Alzheimer’s by disrupting communication between neurons.
How does microglial research contribute to understanding neurodegenerative disorders?
Microglial research plays a pivotal role in unraveling the complexities of neurodegenerative disorders like Alzheimer’s and Huntington’s diseases. By studying how microglial cells interact with neurons and conduct synaptic pruning, researchers like Beth Stevens are uncovering mechanisms that lead to disease, paving the way for new biomarkers and therapeutic options to address these debilitating conditions.
What is synaptic pruning, and how is it related to microglial function?
Synaptic pruning is a natural process where excess synapses in the brain are eliminated to enhance neural efficiency. Microglia facilitate this process by identifying and removing weak or unused synaptic connections. In diseases such as Alzheimer’s, dysregulation of this pruning can result in cognitive decline, highlighting the importance of microglial health in maintaining overall brain function.
Who is Beth Stevens, and what is her contribution to microglial research?
Beth Stevens is a neuroscientist at Harvard Medical School whose research focuses on microglial cells and their role in the brain’s immune system. Her groundbreaking work has reshaped the understanding of how microglia contribute to synaptic pruning and their involvement in neurodegenerative disorders like Alzheimer’s disease. Stevens’ findings are pivotal for developing new treatments and biomarkers for these conditions.
What discoveries in microglial research have implications for Alzheimer’s treatment?
Recent discoveries in microglial research, particularly those led by Beth Stevens, have highlighted how dysregulated microglial activity can contribute to Alzheimer’s disease. By understanding these mechanisms, researchers aim to develop therapies that restore proper microglial function, potentially slowing the progression of Alzheimer’s and improving outcomes for affected individuals.
| Key Point | Description |
|---|---|
| Role of Microglia | Elements of the brain’s immune system that patrol for illness or injury. |
| Impact on Neurodegenerative Diseases | Aberrant pruning by microglia contributes to Alzheimer’s, Huntington’s, and other disorders. |
| Research Foundation | Basic and curiosity-driven research funded by NIH is essential in this field. |
| Potential for Biomarkers and Treatments | Studies led to new biomarkers and potential medicines for Alzheimer’s disease. |
| Basic Science and Human Application | Research in animals can provide insights applicable to human diseases. |
Summary
Microglial research is a critical frontier in neuroscience aimed at understanding how these immune cells function in the brain. As elucidated by Beth Stevens, microglia play a pivotal role in maintaining brain health, but their aberrant activity can lead to devastating neurodegenerative conditions. This research, supported by extensive funding from the National Institutes of Health, not only sheds light on basic science but also paves the way for developing effective biomarkers and treatments for conditions like Alzheimer’s disease. The ongoing exploration into microglial functions continues to promise new insights and potential therapies that could substantially improve the lives of millions affected by these debilitating disorders.